Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Among the following how many molecules have multiply bonds OCl(2), H...
Text Solution
|
- Increasing order of bond angle of (Cl(2)O, ClO(2), Cl(2)O(7), I(3)^(ɵ)...
Text Solution
|
- Which of the following reactions correctly represents the existence of...
Text Solution
|
- How many of the following have zero dipole moment? C Cl(4),,C(2)O(3...
Text Solution
|
- How many number of triatomic species are hypovalent ? CH(3)^(oplus), C...
Text Solution
|
- CaOCl(2)से Cl(2) कैसे प्राप्त करेंगे ?
Text Solution
|
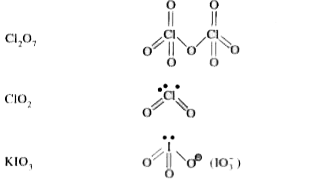
- Cl(2)O, ClO(2) तथा Cl(2)O(7) की संरचनाएँ लिखिए।
Text Solution
|
- The reaction: ClO(3)^(-) +I(2) to IO(3)^(-)+Cl(2)
Text Solution
|
- Arrange the following as indicated below : F(2), Cl(2), Br(2), I(2) ...
Text Solution
|