Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- No. of lone pairs in I bar(Cl(2))
Text Solution
|
- Just like H(2)O, there are two lone pairs in Cl(2)O molecule. But bond...
Text Solution
|
- In a molecule, central atom 'A' has sp^(3) d hybridisation and is surr...
Text Solution
|
- Cl(2) अणु में प्रत्येक Cl परमाणु पर इलेक्ट्रॉनों का केवल एक एकाकी यु...
Text Solution
|
- The space model which is obtained by joining the points representing v...
Text Solution
|
- The total number of lone pairs of electrons present in Cl(2)O(7) molec...
Text Solution
|
- Total no of lone pairs in all atoms of Cl(2)O .
Text Solution
|
- No. of lone pairs in I bar(Cl(2))
Text Solution
|
- Repulsion between bond pair-bond pair is less than in between lone pai...
Text Solution
|
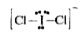 , Total electron pairs `= (7+2+1)/(2) = 5`, Lone pairs = Total -b.p.s = 5-2 =3
, Total electron pairs `= (7+2+1)/(2) = 5`, Lone pairs = Total -b.p.s = 5-2 =3