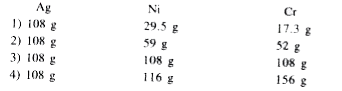Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
AAKASH SERIES|Exercise LEVEL-II (LECTURE SHEET ( EXERCISE -I ( SINGLE & ONE OR MORE THAN ONE CORRECT ANSWER )))|32 VideosELECTROCHEMISTRY
AAKASH SERIES|Exercise LEVEL-II (LECTURE SHEET ( EXERCISE -II (LINKED COMPREHENSION TYPE QUESTIONS )) )|12 VideosELECTROCHEMISTRY
AAKASH SERIES|Exercise LEVEL-I ( EXERCISE-I)|146 VideosDILUTE SOLUTIONS
AAKASH SERIES|Exercise EXERCISE - 1.2|55 VideosELEMENTS OF D - BLOCK
AAKASH SERIES|Exercise EXERCISE - 5.2|33 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ELECTROCHEMISTRY -LEVEL-I ( EXERCISE-II)
- One coulomb of charge passes through solutions of AgNO3 and CuSO4. The...
Text Solution
|
- When electricity is passed through molten AlCl3, 13.5 g. of Al is depo...
Text Solution
|
- One Faraday charge was passed through the electrolytic cell placed in ...
Text Solution
|
- The weight in grams of O2 formed at Pt anode during the electrolys of ...
Text Solution
|
- When one ampere current is passed through a Cu wire for 10 sec., the n...
Text Solution
|
- How many coulombs of electricity are required for the reduction of 1 m...
Text Solution
|
- Electric charge on 1gm ion of N^(3-) is
Text Solution
|
- The charge required for the oxidation of one mole of Mn3O4 " to " MnO...
Text Solution
|
- Time required to deposit one millimole of aluminium metal by the passa...
Text Solution
|
- Two electrolytic cells, one containing acidified ferrous sulphate and ...
Text Solution
|
- On passing a current through a KCl solution, 19.5g of potassium is dep...
Text Solution
|
- When an electric current is passed through acidulated water, 112 ml of...
Text Solution
|
- The amount of chlorine evolved when 2 amperes of current is passed for...
Text Solution
|
- The conductivity of 0.001 M acetic acid is 5 xx 10^(-5) S cm^(-1) and ...
Text Solution
|
- The distance between two electrodes of a cell is 2.5 cm and area of ea...
Text Solution
|
- The limiting molar conductivities (Lambda^(0)) for NaCl, KBr and KCl a...
Text Solution
|
- Which of the following solutions of NaCl has the higher specific condu...
Text Solution
|
- Molar conductivity of a solution is 1.26 xx 10^(2)Omega^(-1) cm^(2) "m...
Text Solution
|
- The values of equivalent conductivity at infinite dilutions for NH4Cl,...
Text Solution
|
- Specific conductance of 0.1 M Nitric acid is 6.3 xx 10^(-2) "ohm"^(-1)...
Text Solution
|