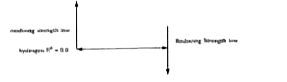
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
AAKASH SERIES|Exercise LEVEL-II (LECTURE SHEET ( EXERCISE -II (LINKED COMPREHENSION TYPE QUESTIONS )) )|12 VideosELECTROCHEMISTRY
AAKASH SERIES|Exercise LEVEL-II (LECTURE SHEET ( EXERCISE -III(MATCH THE FOLLOWING QUESTIONS )))|6 VideosELECTROCHEMISTRY
AAKASH SERIES|Exercise LEVEL-I ( EXERCISE-II)|51 VideosDILUTE SOLUTIONS
AAKASH SERIES|Exercise EXERCISE - 1.2|55 VideosELEMENTS OF D - BLOCK
AAKASH SERIES|Exercise EXERCISE - 5.2|33 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ELECTROCHEMISTRY -LEVEL-II (LECTURE SHEET ( EXERCISE -I ( SINGLE & ONE OR MORE THAN ONE CORRECT ANSWER )))
- Which of the following facts are true ?
Text Solution
|
- In a lead storage battery
Text Solution
|
- Which one is correct among the following give, the half cell emf's E^...
Text Solution
|
- For the cell (at 298 K ) Ag((g)) // AgCl((s)) // Cl((aq))^(-) ||AgNO(3...
Text Solution
|
- The value of the reaction quotient ,Q for the cell Zn(s) |Zn^(2+) (0.0...
Text Solution
|
- DeltaG = DeltaH-TdeltaS and DeltaG = DeltaH + T[(d(DeltaG))/(dT)] " ...
Text Solution
|
- For the cell Zn|Zn^(2+) (C1)||Zn^(2+) (C2)|Zn. DeltaG is negative if :
Text Solution
|
- The Nernst equation , E = E^(0) -(RT)/(nF) . In Q indicates that the ...
Text Solution
|
- The half cell reaction for the corrosion are , 2H^(+) + (1)/(2) O2 + ...
Text Solution
|
- The E^(@) at 25^(@)C for the following reaction at the indicated conc...
Text Solution
|
- A fuel cell develops an electrical potential from the combustion of b...
Text Solution
|
- For a cell reaction 2H(2(g)) + O(2(g)) to 2H2O((l)) DeltaS(298)^(@) ...
Text Solution
|
- Copper reduces NO3^(-) into NO and NO2 depending upon concentration of...
Text Solution
|
- Consider the following standard reduction potentials Fe^(3+)(aq) + e...
Text Solution
|
- Which of the following statements is / are correct ?
Text Solution
|
- Which of the following facts regarding the movement of anions in the s...
Text Solution
|
- Which of the following statements is correct ? If E(Cu^(2+)|Cu)^(@)...
Text Solution
|
- The oxidation potential of hydrogen half-cell will be negative if :
Text Solution
|
- Coulomb is the quantity of charge defined as :
Text Solution
|
- E^(@) for two reactions are given below : Cr^(+3) + 3e^(-) to Cr , E^(...
Text Solution
|