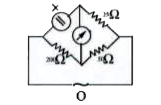
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
AAKASH SERIES|Exercise PRACTICE SHEET -5 (LINKED COMPREHENSION TYPE QUESTIONS (PASSAGE-I))|3 VideosELECTROCHEMISTRY
AAKASH SERIES|Exercise PRACTICE SHEET -5 (LINKED COMPREHENSION TYPE QUESTIONS (PASSAGE-II))|3 VideosELECTROCHEMISTRY
AAKASH SERIES|Exercise PRACTICE SHEET -4 (INTEGER ANSWER TYPE QUESTIONS )|6 VideosDILUTE SOLUTIONS
AAKASH SERIES|Exercise EXERCISE - 1.2|55 VideosELEMENTS OF D - BLOCK
AAKASH SERIES|Exercise EXERCISE - 5.2|33 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ELECTROCHEMISTRY -PRACTICE SHEET -5 (SINGLE OR MORE THAN ONE OPTION QUESTIONS)
- The E^(@) at 25^(@)C for the following reaction at the indicated conc...
Text Solution
|
- The standard electrode potential for the following reaction is + 1.33V...
Text Solution
|
- The equilibrium constant for the following general reaction is 10^(@)...
Text Solution
|
- If E(Au^(+)// Au)^(@) is 1.69 V and E(Au^(3+)//Au)^@ is 1.40 V . The...
Text Solution
|
- The molar conductivities of H^(+) and HCOO^(-) ions at infinite diluti...
Text Solution
|
- Limiting equivalent conductivities of different ions are given below ...
Text Solution
|
- lambdam^(@)(AgNO3), lambdam^(@)(HCl) and lambdam^(@)(HNO3) are a,b and...
Text Solution
|
- A conductivity cell (X) employs two electrodes having surface area of ...
Text Solution
|
- Aqueous solution of AgNO3 is electrolysed using inert electrodes. At t...
Text Solution
|
- An aqueous solution containing one mole per litre of each Cu(NO3)2 , A...
Text Solution
|
- Which statements is/are correct ?
Text Solution
|
- Which of the following statements are true ?
Text Solution
|
- Fused AlF3 and fused NaF are electrolysed in a series of cells with sa...
Text Solution
|
- In hydrogen fule cell
Text Solution
|
- In the following concentration cell Ag(s) // AgCl "(saturated)"// //...
Text Solution
|
- Standared electrode potential of two half-reactions are given below : ...
Text Solution
|