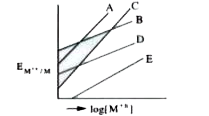
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
AAKASH SERIES|Exercise PRACTICE SHEET -5 (MATCH THE FOLLOWING QUESTIONS )|2 VideosELECTROCHEMISTRY
AAKASH SERIES|Exercise PRACTICE SHEET -5 (INTEGER ANSWER TYPE QUESTIONS )|6 VideosELECTROCHEMISTRY
AAKASH SERIES|Exercise PRACTICE SHEET -5 (LINKED COMPREHENSION TYPE QUESTIONS (PASSAGE-I))|3 VideosDILUTE SOLUTIONS
AAKASH SERIES|Exercise EXERCISE - 1.2|55 VideosELEMENTS OF D - BLOCK
AAKASH SERIES|Exercise EXERCISE - 5.2|33 Videos
Similar Questions
Explore conceptually related problems