A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AAKASH SERIES-SOLID STATE-PRACTICE SHEET - 5
- In rock salt structure, what percentage of the octahedral sites are oc...
Text Solution
|
- Determine the simplest formula of an ionic compound in which cations p...
Text Solution
|
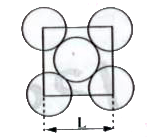
- The packing efficiency of the two-dimensional square unit cell shown b...
Text Solution
|
- What is the co-ordination number of sodium in Na(2)O ?
Text Solution
|
- Total volume of atoms present in a face centered cubic unit cell of a ...
Text Solution
|
- In b.c.c structure of lattice constant 'a' the minimum distance betwee...
Text Solution
|
- In a cubic cell, seven of the eight corners are occupied by atoms A an...
Text Solution
|
- A TV in FCC is formed by atoms at
Text Solution
|
- Which planes can be found in a bcc unit cell?
Text Solution
|
- Which planes can be found in fluorite structure (unit cell)?
Text Solution
|
- Which planes are present in Na(2)O structure (unit cell)
Text Solution
|
- Which of the following solids fuse at modulate temperature ?
Text Solution
|
- Which systems have more than one unit cell ?
Text Solution
|
- Which statements are correct ?
Text Solution
|
- Rocksalt structure consists of FCC arrangment of Cl^(-) ions in which ...
Text Solution
|
- Rocksalt structure consists of FCC arrangment of Cl^(-) ions in which ...
Text Solution
|
- Rocksalt structure consists of FCC arrangment of Cl^(-) ions in which ...
Text Solution
|
- In Zns structure S^(-2) ions make FCC lattice in which Zn^(+) occupies...
Text Solution
|
- In Zns structure S^(-2) ions make FCC lattice in which Zn^(+) occupies...
Text Solution
|
- If CaF(2) structure the nearest inter cationic distance in 'x' what is...
Text Solution
|