A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SURFACE CHEMISTRY
AAKASH SERIES|Exercise PRACTICE SHEET - 3 (LINKED COMPREHENSION TYPE QUESTIONS)|6 VideosSURFACE CHEMISTRY
AAKASH SERIES|Exercise PRACTICE SHEET - 3 (MATCH THE FOLLOWING QUESTIONS)|2 VideosSURFACE CHEMISTRY
AAKASH SERIES|Exercise PRACTICE SHEET - 2 (INTEGER ANSWER TYPE QUESTIONS)|6 VideosSTOICHIOMETRY
AAKASH SERIES|Exercise PRACTICE SHEET (ADVANCED) (INTEGER TYPE QUESTION)|2 VideosVA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE EXERCISE|51 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-SURFACE CHEMISTRY-PRACTICE SHEET - 3 (SINGE OR MORE THAN ONE OPTION QUESTIONS)
- A sample of 16gr of charcoal was brought into contact with CH4 gas con...
Text Solution
|
- 10% sites of catalyst bed have adsorbed by H2 on heating H2 gas is evo...
Text Solution
|
- When a solution of acetic acid in water is shaken with charcoal
Text Solution
|
- Which is correct statement regarding enzyme catalysis
Text Solution
|
- Which of the following are correct A) T3 >T2 > T1 B) At fixed ...
Text Solution
|
- In freundlich adsorption isotherm, the value of 1/n is
Text Solution
|
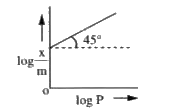
- Plot of "log" x/m against log p is a straight line inclined at an angl...
Text Solution
|
- The volumes of gases H2,CH4,CO2 and NH3 adsorbed by 1gr of charcoal a...
Text Solution
|
- Which of the following is lyophobic colloidol solution?
Text Solution
|
- The catalyst used in the manufacture of H2 by Bosche's process is
Text Solution
|
- Assosiated colloids
Text Solution
|
- The emulsifier added to equal quantity of oil and water has the affini...
Text Solution
|
- What are correct statements ?
Text Solution
|
- Which statements are correct ?
Text Solution
|
- Which statements are correct ?
Text Solution
|
- Which statements are correct?
Text Solution
|