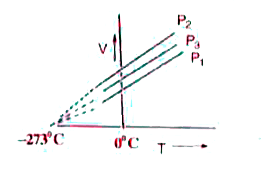A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER
AAKASH SERIES|Exercise LECTURE SHEET - EXERCISE - I (LEVEL - II ADVANCED)(STRAIGHT OBJECTIVE TYPES QUESTIONS)|11 VideosSTATES OF MATTER
AAKASH SERIES|Exercise LECTURE SHEET - EXERCISE - I (LEVEL - II ADVANCED)(MORE THAN ONE CORRECT ANSWER TYPES QUESTIONS)|5 VideosSATURATED HYDROCARBONS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|10 VideosSTOICHIOMETRY
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|59 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-STATES OF MATTER-OBJECTIVE EXERCISE -3
- The volume-temperature graphs of a given mass of an ideal gas at const...
Text Solution
|
- Van der Waal's real gas, acts as an ideal gas, at which conditions
Text Solution
|
- Volume occupied by one molecule of water (density = 1g cm^(-3)) is
Text Solution
|
- If a gas expands at constant temperature.
Text Solution
|
- The energy absorbed by each molecule (A2) of a substance is 4.4 xx 10^...
Text Solution
|
- The pressure exerted by 6.0 g fo methane gas in 0.03m^3 vessel at 129^...
Text Solution
|
- A bubble of air is underwater at temperature 15^@ C and the pressure 1...
Text Solution
|
- A gaseous mixture was prepared by taking equal mole of CO and N2 If th...
Text Solution
|
- Two gases A and B having the same volume diffuse through a porous part...
Text Solution
|
- By what factor does the average velocity of a gaseous molecule increas...
Text Solution
|
- Equal volumes of two monatomic gases, A and B at same temperature and ...
Text Solution
|
- For real gases van der Waals equation is written as (P + (an^2)/(V^2))...
Text Solution
|
- A certain gas takes three times as long to effuse out as helium. Its m...
Text Solution
|
- What is the density of N2 gas at 227^@ C and 5.00atm. pressure? (R = 0...
Text Solution
|
- Maximum deviation from ideal gas is expected from
Text Solution
|
- Dipole-induced dipole interactions are present in which of the followi...
Text Solution
|
- Equal masses of H2, O2 and methane have been taken in a container of v...
Text Solution
|
- A gas such as carbon monoxide would be most likely to obey the ideal g...
Text Solution
|
- Equal moles of hydrogen and oxygen gases are placed in a container wit...
Text Solution
|
- HgCl2 and I2 both when dissolved in water containing I^(-) ions the pa...
Text Solution
|