A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER
AAKASH SERIES|Exercise LECTURE SHEET - EXERCISE - II (LEVEL - II ADVANCED)(MORE THAN ONE CORRECT ANSWER TYPES QUESTIONS)|3 VideosSTATES OF MATTER
AAKASH SERIES|Exercise LECTURE SHEET - EXERCISE - II (LEVEL - II ADVANCED)(MATRIX MATCHING TYPE QUESTIONS)|1 VideosSTATES OF MATTER
AAKASH SERIES|Exercise LECTURE SHEET - EXERCISE - II (LEVEL - I MAIN)(STRAIGHT OBJECTIVE TYPES QUESTIONS)|24 VideosSATURATED HYDROCARBONS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|10 VideosSTOICHIOMETRY
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|59 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-STATES OF MATTER-LECTURE SHEET - EXERCISE - II (LEVEL - II ADVANCED)(STRAIGHT OBJECTIVE TYPES QUESTIONS)
- In a mixture of N2 and CO2 gases, the partial pressure of CO(2) is 1....
Text Solution
|
- Equal volumes of two jars contain HCI, NH3 gases respectively at const...
Text Solution
|
- When 2g of a gas A is introduced into an evacuated flask kept at 25°C,...
Text Solution
|
- A vessel has N2 gas saturated with water vapour at a total pressure of...
Text Solution
|
- The reaction between gaseous NH3 and HBr produces a white solid NH4Br....
Text Solution
|
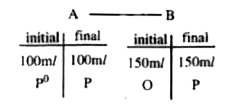
- Two glass bulbs A and B are connected by very small tube having a stop...
Text Solution
|
- 10 Its of an air sample with relative humidity 0.6 is compressed to 5 ...
Text Solution
|
- Two containers A and B have the same volume. Container 'A' contains 5 ...
Text Solution
|