A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER
AAKASH SERIES|Exercise LECTURE SHEET - EXERCISE - III (LEVEL - II ADVANCED)(MATRIX MATCHING TYPE QUESTIONS)|1 VideosSTATES OF MATTER
AAKASH SERIES|Exercise LECTURE SHEET - EXERCISE - III (LEVEL - II ADVANCED)(INTEGER TYPE QUESTIONS)|4 VideosSTATES OF MATTER
AAKASH SERIES|Exercise LECTURE SHEET - EXERCISE - III (LEVEL - II ADVANCED)(MORE THAN ONE CORRECT ANSWER TYPE QUESTIONS)|7 VideosSATURATED HYDROCARBONS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|10 VideosSTOICHIOMETRY
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|59 Videos
AAKASH SERIES-STATES OF MATTER-LECTURE SHEET - EXERCISE - III (LEVEL - II ADVANCED)(LINKED COMPREHENSION TYPE QUESTIONS)
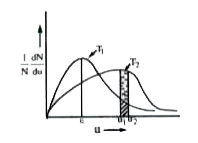
- According to Maxwell distribution, the area under the curve is equal t...
Text Solution
|
- According to Maxwell distribution, the area under the curve is equal t...
Text Solution
|
- According to Maxwell distribution, the area under the curve is equal t...
Text Solution
|
- The root mean square speed of an ideal gas is given by : u("rms") = sq...
Text Solution
|
- The root mean square speed of an ideal gas is given by : u("rms") = sq...
Text Solution
|
- The root mean square speed of an ideal gas is given by : u("rms") = sq...
Text Solution
|