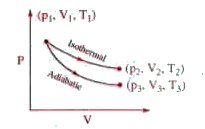A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE-I) (LEVEL - II (ADVANCED) LINKED COMPREHENSION TYPE QUESTIONS)|5 VideosCHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE-I) (LEVEL - II (ADVANCED) MATRIX MATCHING TYPE QUESTIONS)|1 VideosCHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE-I) (LEVEL - II (ADVANCED) STRAIGHT OBJECTIVE TYPE QUESTIONS)|5 VideosCHEMICAL THERMODYANMICS
AAKASH SERIES|Exercise Questions For Descriptive Answers|28 VideosELECTRON MIGRATION EFFECTS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|10 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMICAL THERMODYNAMICS-LECTURE SHEET (EXERCISE-I) (LEVEL - II (ADVANCED) MORE THAN ONE CORRECT ANSWER TYPE QUESTIONS)
- The following is/are extensive property/properties
Text Solution
|
- If x and Y are two intensive variables then:
Text Solution
|
- During the isothermal expansion of an ideal gas
Text Solution
|
- Which of the following is/are true in the case of an adiabatic process
Text Solution
|
- The internal energy of an ideal gas decreases by the same amount as th...
Text Solution
|
- For the adiabatic expansion of an ideal gas
Text Solution
|
- In a process a system does 140j of work on the surroundings and only 4...
Text Solution
|
- If a gas absorbs 200J of heat and expands by 500 cm^(3) against a cons...
Text Solution
|
- 1g H(2) gas at STP is expanded so that volume is doubled. Hence, work ...
Text Solution
|
- 1 mole of an ideal gas A (C(v,m)=3R) and 2 mole of an ideal gas B are ...
Text Solution
|
- Two mole of an ideal gas is heated at constant pressure of one atmosph...
Text Solution
|
- The reversible expansion of an ideal gas under adiabatic and isotherma...
Text Solution
|