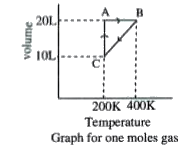
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE-I) (LEVEL - II (ADVANCED) MATRIX MATCHING TYPE QUESTIONS)|1 VideosCHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE-I) (LEVEL - II (ADVANCED) INTEGER TYPE QUESTIONS)|4 VideosCHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE-I) (LEVEL - II (ADVANCED) MORE THAN ONE CORRECT ANSWER TYPE QUESTIONS)|12 VideosCHEMICAL THERMODYANMICS
AAKASH SERIES|Exercise Questions For Descriptive Answers|28 VideosELECTRON MIGRATION EFFECTS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|10 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMICAL THERMODYNAMICS-LECTURE SHEET (EXERCISE-I) (LEVEL - II (ADVANCED) LINKED COMPREHENSION TYPE QUESTIONS)
- Process A rarr B represents
Text Solution
|
- Work done in the process C rarr A is
Text Solution
|
- The process which occurs in going from B rarr C is
Text Solution
|
- One mole of an ideal gas for which C(V)= 3//2R heated at a constant pr...
Text Solution
|
- One mole of an ideal gas for which C(V)= 3//2R heated at a constant pr...
Text Solution
|