A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE- II) (LEVEL- II (ADVANCED) MATRIX MATCHING TYPE QUESTIONS)|2 VideosCHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE- II) (LEVEL- II (ADVANCED) INTEGER TYPE QUESTIONS)|6 VideosCHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE- II) (LEVEL- II (ADVANCED) MORE THAN ONCE CORRECT ANSWER TYPE QUESTIONS)|6 VideosCHEMICAL THERMODYANMICS
AAKASH SERIES|Exercise Questions For Descriptive Answers|28 VideosELECTRON MIGRATION EFFECTS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|10 Videos
AAKASH SERIES-CHEMICAL THERMODYNAMICS-PRACTICE SHEET (EXERCISE- II) (LEVEL- II (ADVANCED) LINKED COMPREHENSION TYPE QUESTIONS)
- Enthalpy of neutralization is defined as the enthalpy change when 1 mo...
Text Solution
|
- Enthalpy of neutralization is defined as the enthalpy change when 1 mo...
Text Solution
|
- Enthalpy of neutralization is defined as the enthalpy change when 1 mo...
Text Solution
|
- The bond dissociation energy depends upon the nature of the bond and n...
Text Solution
|
- The bond dissociation energy depends upon the nature of the bond and n...
Text Solution
|
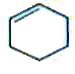 is ? Answer `Delta H_("vap") " of " C_(6)H_(6(l)), C_(6)H_(10(l)), C_(6)H_(12(l))` all are equal:
is ? Answer `Delta H_("vap") " of " C_(6)H_(6(l)), C_(6)H_(10(l)), C_(6)H_(12(l))` all are equal: