A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The number and type of bonds between two carbon atoms in CaC(2) are
Text Solution
|
- The number and type of bonds between two carbon atoms in CaC(2) are:
Text Solution
|
- The number and type of bonds between two carbon atoms in CaC(2) are:
Text Solution
|
- The number and type of bonds between two carbon atoms in calcium carbi...
Text Solution
|
- Number and type of bonds between two carbon atoms in CaC(2) are :
Text Solution
|
- The number and type of bonds between two carbon atoms in C(2) are:
Text Solution
|
- CaC(2) में दो कार्बन परमाणुओं के बीच बंधो का प्रकार तथा उनकी संख्या है
Text Solution
|
- The number and type of bonds between two carbon atoms in CaC(2) are:
Text Solution
|
- Number and type of bonds between two carbon atoms in CaC(2) are :
Text Solution
|
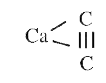 one `sigma` and two `pi` bonds.
one `sigma` and two `pi` bonds.