A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC CLASSIFICATION
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 1 (ELECTRONEGATIVITY)|15 VideosPERIODIC CLASSIFICATION
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 1 (VALENCY AND OXIDATION STATES)|8 VideosPERIODIC CLASSIFICATION
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 1 (IONISATION POTENTIAL)|25 VideosISOMERISM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE ( PRACTICE SHEET (ADVANCED) INTEGER TYPE QUESTIONS)|10 VideosPERIODIC PROPERTIES
AAKASH SERIES|Exercise LEVEL-II (TYPE -II) (PRACTICE SHEET -3 (SUBJECTIVE/ ANALYTICAL TYPE QUESTIONS ))|8 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-PERIODIC CLASSIFICATION-OBJECTIVE EXERCISE - 1 (ELECTRON AFFINITY)
- Electron affinity is
Text Solution
|
- Electron affinity is measured in
Text Solution
|
- The element with highest electron affinity is
Text Solution
|
- Which of the following is true about the element with atomic number 18...
Text Solution
|
- Ionisation of energy of F^(-) is 320 kJ mol^(-1) . The electron gain e...
Text Solution
|
- The electron affinity values of elements A,B, C and D are respectively...
Text Solution
|
- Which of the following is an endothermic process?
Text Solution
|
- In a period from left to right, electron affinity
Text Solution
|
- For the process X((g)) + e^(-) rarr X((g))^(-), Delta H =x and X(g)^...
Text Solution
|
- Configuration that shows the highest energy released when an electron ...
Text Solution
|
- Electron affinity of Fluorine is less than that of Chlorine because
Text Solution
|
- Among chalcogens electron affinity is highest for
Text Solution
|
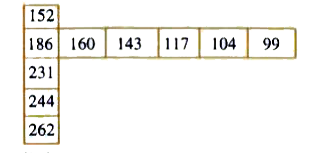
- Few values are given in the table in the direction from left to right ...
Text Solution
|