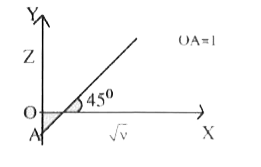
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC CLASSIFICATION
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 2 (ATOMIC RADIUS)|15 VideosPERIODIC CLASSIFICATION
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 2 (IONISATION POTENTIAL)|23 VideosPERIODIC CLASSIFICATION
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 1 (DIAGONAL RELATIONSHIP)|6 VideosISOMERISM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE ( PRACTICE SHEET (ADVANCED) INTEGER TYPE QUESTIONS)|10 VideosPERIODIC PROPERTIES
AAKASH SERIES|Exercise LEVEL-II (TYPE -II) (PRACTICE SHEET -3 (SUBJECTIVE/ ANALYTICAL TYPE QUESTIONS ))|8 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-PERIODIC CLASSIFICATION-OBJECTIVE EXERCISE - 2 (PERIODIC CLASSIFICATION)
- The atomic number of an element is 58. it blongs to
Text Solution
|
- In the periodic table, inversion of atomic weights took place in this ...
Text Solution
|
- The frequency of the characterstic X ray of K(alpha) line of metal tar...
Text Solution
|
- The period that contains only gaseous elements is
Text Solution
|
- Pair of elements with the following atomic numbers have the same chemi...
Text Solution
|
- The sub-shells filled one by one for 4th period elements are
Text Solution
|
- The starting element and last element in the largest period in modern ...
Text Solution
|
- Which of the following has both members from the same period of the pe...
Text Solution
|
- As per the modern periodic law, the physical and chemical properties o...
Text Solution
|
- Atomic number of nitrogen is 7. The atomic number of the third member ...
Text Solution
|
- Element with atomic number 38, belongs to
Text Solution
|
- Set of elements with the following atomic numbers belong to the same g...
Text Solution
|
- The element which belong to 3rd period and IVA group of periodic table...
Text Solution
|
- Screening by inner electrons will be more effective in
Text Solution
|
- Which of the following pairs has elements containing same number of el...
Text Solution
|
- The period that includes all blocks of elements is
Text Solution
|
- Among s-block metals and transition metals, which are more metallic?
Text Solution
|
- Element with atomic number 52 belongs to
Text Solution
|
- The period number and group number in which maximum number of elements...
Text Solution
|
- The general electronic configuration of f-block elements is
Text Solution
|