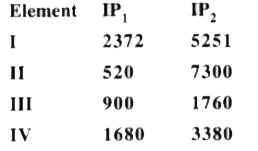A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC TABLE
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -3|30 VideosPERIODIC TABLE
AAKASH SERIES|Exercise EXERCISE ON PASSAGE|12 VideosPERIODIC TABLE
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -2A (DIAGONAL RELATIONSHIP)|8 VideosPERIODIC PROPERTIES
AAKASH SERIES|Exercise LEVEL-II (TYPE -II) (PRACTICE SHEET -3 (SUBJECTIVE/ ANALYTICAL TYPE QUESTIONS ))|8 VideosPURIFICATION OF ORGANIC COMPOUNDS AND IUPAC NOMENCLATURE
AAKASH SERIES|Exercise ADDITIONAL PRCATICE EXERCISE (LEVEL - II (LECTURE SHEET (ADVANCED) INTEGER TYPE QUESTIONS)|2 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-PERIODIC TABLE-OBJECTIVE EXERCISE -2B
- Consider the following changes a) M((g))^(+2) rarr M((g))^(+3) + e^...
Text Solution
|
- The correct order of IP2 , values among F, Na, Mg and Al is
Text Solution
|
- The first (IP1) and the second (IP2) ionisation potential (kJ mol 1) o...
Text Solution
|
- The ionisation energies of Lithium and Sodium are 520kJ Mol^(-1) and 4...
Text Solution
|
- Successive ionisation potentials of an element M are 8.3, 25.1, 37.9, ...
Text Solution
|
- The first ionisation potentials of four consecutive elements present i...
Text Solution
|
- H-H, X-X and H-X bond energies are 104 Kcal/mole 60Kcal/mole and 101Kc...
Text Solution
|
- The ionisation energy and electron affinity of an element are 13.0ev a...
Text Solution
|
- If the ionisation energy and electron affinity of an element are 275 a...
Text Solution
|
- The elements which show both positive and negative oxidation states. i...
Text Solution
|
- Match the following two lists given below in view of higest oxidation ...
Text Solution
|
- Strongest reducing agent and strongest oxidising agent are respectivel...
Text Solution
|
- Which of the following statement is correct
Text Solution
|
- The radius of La^(3+) (Z=57) is 1.06A^(@). Which one of the following ...
Text Solution
|
- (A): According to Mendeleeff atomic weight of 'Be' is 9.1 but experime...
Text Solution
|
- Chloride of an element A gave a neutral solution in water. In the peri...
Text Solution
|
- Which one of the following relations is correct with respect to first ...
Text Solution
|
- The incorrect statement among the following is
Text Solution
|
- The decreasing order of second ionisation potential of K, Ca, Ba is
Text Solution
|
- For four elements the IP2 curve is shown. In the graph the elements re...
Text Solution
|