Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AAKASH SERIES-STOICHIOMETRY-PROBLEMS
- An organic compound contains carbon, hydrogen, oxygen and nitrogen in ...
Text Solution
|
- Combustion of 0.277g of an organic compound gave 0.66g carbondioxide a...
Text Solution
|
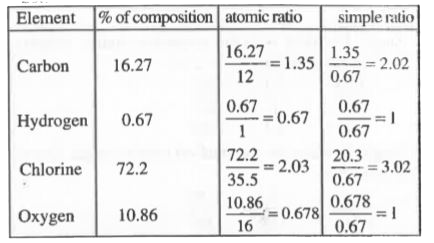
- An organic compound on analysis was found to contain 16.27% carbon. 0....
Text Solution
|
- Combustion of 0.202 g of a carbon compound gave 0.361 g of carbon diox...
Text Solution
|
- The weight ratio of elements carbon, nitrogen and hydrogen in a compou...
Text Solution
|
- A carbon compound contains 12.8% Carbon, 2.1% Hydrogen, 85.1% Bromine....
Text Solution
|
- The empirical formula an organic substance is CH(2)O. If 3 xx 10^(22) ...
Text Solution
|
- Combustion of 0.277g of an organic compound gave 0.66g carbondioxide a...
Text Solution
|
- Justify that the reaction : 2Cu(2) O(s) + Cu(2)S (s) rarr 6 Cu (s) + S...
Text Solution
|
- Can oxygen exhibit positive oxidation numbers in its compounds?
Text Solution
|
- What is the oxidation state of chromium in potassium dichromate?
Text Solution
|
- What are the oxidation numbers of : (a) S in H(2)SO(4), (b) Mn in MnO(...
Text Solution
|
- Write the oxidation number of oxygen in (a) O(3), (b) MgO, (c) H(2)O(2...
Text Solution
|
- Calculate the oridation numbers of sulphur in H(2)SO(5) and in H(2)S(2...
Text Solution
|
- Using stock notation represent HAuCI(4).
Text Solution
|
- What is the oxidation number of iron in the brown ring complex compoun...
Text Solution
|
- Oxidation number of the metal ion in the compound [CO(NH(3))(5)Cl]Cl(2...
Text Solution
|
- One mole of hydrazine loses 10 moles of electrons. If all the nitrogen...
Text Solution
|
- Mention whether each of the following conversion involves oxidation or...
Text Solution
|
- One mole of AO(2)^(-) is oxidised to A^(n+) in acidic solutions by 0.4...
Text Solution
|