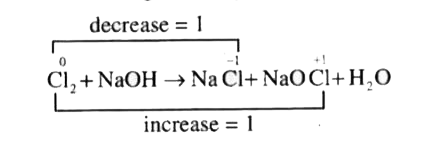Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AAKASH SERIES-STOICHIOMETRY-QUESTIONS FOR DESCRIPTIVE ANSWERS
- Chlorine reacts with cold dilute caustic soda to give sodium chloride,...
Text Solution
|
- Distinguish between element and compound?
Text Solution
|
- Copper forms two oxide Cu(2)O(x) and Cu(2)O(y). For the same amount of...
Text Solution
|
- What are the molecular mass and molar mass, if each molecule of a subs...
Text Solution
|
- Density of metal is 7.42 ""gcc""^(-1). If the radius of metal atom is ...
Text Solution
|
- Calculate the mass of 10^(22) formula units of blue vitriol.
Text Solution
|
- The atomic weight of two isotopes of boron are 10.01 and 11.01 . If th...
Text Solution
|
- Ratio of atoms in an oxide of sulphur is 3:1 .What is the ratio of wei...
Text Solution
|
- There are more number of atoms in one gram atom of an element than in ...
Text Solution
|
- The formula weight of metal chloride is 136 and specific gravity of me...
Text Solution
|
- How many fundamental particles are present in 10u of helium?
Text Solution
|
- 0.22g of a metal chloride required 0.51g of AgNO(3) to precipitate chl...
Text Solution
|
- Density of a gas relative to air is 1.17. What is the molar mass of ga...
Text Solution
|
- KCl contains 52% of potassium and KI contains 23.6% potassium by weigh...
Text Solution
|
- What weight of flurine is present in one gram of a fluoride tooth past...
Text Solution
|
- One gram of copper is dissolved in nitric acid and crystal hydrate Cu(...
Text Solution
|
- The weight ratio of elements carbon, nitrogen and hydrogen in a compou...
Text Solution
|
- 100grams of a hydrocarbon has 93.71 g of carbon. What is its empirical...
Text Solution
|
- 0.234g of an organic compound on heating with conc nitric acid and sil...
Text Solution
|
- An organic compound contains 52.2%C, 13%H and remaining oxygen.What is...
Text Solution
|
- Two oxides of a non-metal X contain 50% and 40% of non -metal respecti...
Text Solution
|