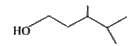
Text Solution
Verified by Experts
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
AAKASH SERIES|Exercise Subjective Exercise -1|11 VideosGENERAL ORGANIC CHEMISTRY
AAKASH SERIES|Exercise Subjective Exercise -2|6 VideosENVIRONMENTAL CHEMISTRY
AAKASH SERIES|Exercise EXERCISE ON PASSAGE (PASSAGE-III)|5 VideosHYDROCARBONS
AAKASH SERIES|Exercise ADDITIONAL PRACTICE SHEET ( LEVEL-II (PRACTICE SHEET (ADVANCED) (MATRIX MATCHING TYPE QUESTIONS)))|5 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-GENERAL ORGANIC CHEMISTRY-QUESTIONS FOR DESCRIPTIVE ANSWERS
- For each of the following compounds, write a condensed formula and al...
Text Solution
|
- Mention the hybridisation stste of each carbon in the following compou...
Text Solution
|
- Indicate the sigma and pi bonds in naphthalene and isobutane molecule...
Text Solution
|
- What type of organic compounds can be purified by sublimation ? Give ...
Text Solution
|
- What is the difference between distillation, distillation under reduce...
Text Solution
|
- Describe the method, which can be used to separate two compounds with ...
Text Solution
|
- Write the equations involved in the detection of Nitrogen, Halogens an...
Text Solution
|
- Nitrogen, sulphur and phosphorus present in organic compounds are dete...
Text Solution
|
- 0.29 g of an organic compound were analysed by Liebig's method. The in...
Text Solution
|
- 0.22g of an organic compound on combustion in an atomosphere of carbon...
Text Solution
|
- 0.303 g of an organic compound was analysed for nitrogen by Kjedahl's ...
Text Solution
|
- 0.246 g of an organic substance when heated with excess of fuming nitr...
Text Solution
|
- In a carius determination, 0.234g of an organic substance gave 0.334g ...
Text Solution
|
- 1.5 g of an organic compound in a quantitative determination of phosph...
Text Solution
|
- Indicate the number of 1^(@), 2^(@), 3^(@) and 4^(@) carbon atoms in t...
Text Solution
|
- Give IUPAC name of the compound. CH(3)-underset(OH)underset(|)CH-under...
Text Solution
|
- Write the structures of the following compounds: 2-Amino - 3 - methylb...
Text Solution
|
- What are the common names of the following compounds ? CH(2)=C=CH(2...
Text Solution
|
- An alkane has molecular mass 86. What is the molecular formula of the ...
Text Solution
|
- Identify molecules containing chirality centre among the following : s...
Text Solution
|
- Assign E and Z configuration to the following structures :
Text Solution
|