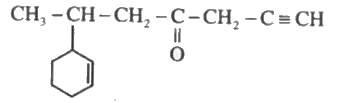Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
AAKASH SERIES|Exercise Subjective Exercise -2|6 VideosGENERAL ORGANIC CHEMISTRY
AAKASH SERIES|Exercise Subjective Exercise -3|17 VideosGENERAL ORGANIC CHEMISTRY
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|35 VideosENVIRONMENTAL CHEMISTRY
AAKASH SERIES|Exercise EXERCISE ON PASSAGE (PASSAGE-III)|5 VideosHYDROCARBONS
AAKASH SERIES|Exercise ADDITIONAL PRACTICE SHEET ( LEVEL-II (PRACTICE SHEET (ADVANCED) (MATRIX MATCHING TYPE QUESTIONS)))|5 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-GENERAL ORGANIC CHEMISTRY-Subjective Exercise -1
- Explain the structures of CH(4) and C(2)H(6).
Text Solution
|
- Discuss the strusture and hybridisation in ethylene.
Text Solution
|
- Explain sp hybridisation taking acethylene as example.
Text Solution
|
- The chemical formula of methane is…………………
Text Solution
|
- What are the main natural sources of organic compounds?
Text Solution
|
- Indicate hybrid state of each carbon atom in the following molecule
Text Solution
|
- Give the order of bond lengths of various hybrid carbon atoms and hydr...
Text Solution
|
- Comment on the bond-line structural representation of hydrocarbons
Text Solution
|
- Write the Lewis structures of methyl nitrite and ethyl alcohol,
Text Solution
|
- Write the bond line formula of cyclopentanol and 2-enthyl -4-methylpen...
Text Solution
|
- Write the IUPAC names of the following compounds
Text Solution
|