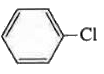
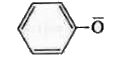
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
AAKASH SERIES|Exercise EXERCISE - 1.1.1|8 VideosGENERAL ORGANIC CHEMISTRY
AAKASH SERIES|Exercise EXERCISE - 1.1.2|4 VideosGENERAL ORGANIC CHEMISTRY
AAKASH SERIES|Exercise Objective Exercise -2|82 VideosENVIRONMENTAL CHEMISTRY
AAKASH SERIES|Exercise EXERCISE ON PASSAGE (PASSAGE-III)|5 VideosHYDROCARBONS
AAKASH SERIES|Exercise ADDITIONAL PRACTICE SHEET ( LEVEL-II (PRACTICE SHEET (ADVANCED) (MATRIX MATCHING TYPE QUESTIONS)))|5 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-GENERAL ORGANIC CHEMISTRY-Practice Exercise
- Primary, secondary and tertiary amines are
Text Solution
|
- Which pair of isomers given below are position isomers?
Text Solution
|
- n-propyl amine and isopropyl amine are examples of
Text Solution
|
- The number of isomeric amines possible for the formula C(3)H(9)N
Text Solution
|
- The type of isomerism that is not found in alkenes is
Text Solution
|
- Which pair does not represent isomers?
Text Solution
|
- Number of isomers for the compound dihydroxy benzene
Text Solution
|
- The number of isomeric structures possible for a molecule having molec...
Text Solution
|
- A pair of functional isomers
Text Solution
|
- Out of the following the alkene that exhibits optical isomerism is
Text Solution
|
- Number of possible geometrical isomers for 2, 4 - hexadjenie is
Text Solution
|
- Removl of hydride ion from a methane molecule will give a
Text Solution
|
- The most stable carbonium ion is
Text Solution
|
- Which one of the following is most stable?
Text Solution
|
- Which of the following is the least stable carbonium ion?
Text Solution
|
- Which is the following shows maximum -I effect?
Text Solution
|
- Which of the following has maximum number of hyper conjugative structu...
Text Solution
|
- The substance stabilized by resonance
Text Solution
|
- What are the shapes of ethyne and methane?
Text Solution
|
- The IUPAC name of the following compound is
Text Solution
|