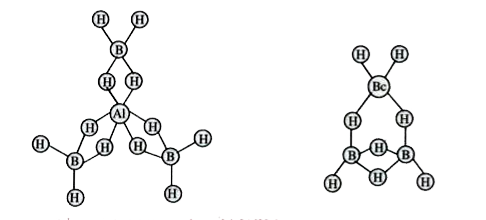
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- NaBH(4) is ionic compound contain BH(4)^(-) tetrahydridoborate ion and...
Text Solution
|
- (All products from P to related to Boron. The by products are not incl...
Text Solution
|
- Number of 3c-2e bonds (hydrogen bridges) in Be(BH(4))(2) is
Text Solution
|
- Solid CuSO(4)*5H(2)O having covalent, ionic as well as co-ordinate bon...
Text Solution
|
- Carbon oxygen double bond are easily reduced by NaBH(4) or LiAlH(4). T...
Text Solution
|
- Carbon oxygen double bond are easily reduced by NaBH(4) or LiAlH(4). T...
Text Solution
|
- Carbon oxygen double bond are easily reduced by NaBH(4) or LiAlH(4). T...
Text Solution
|
- Carbon-oxygen double bond are easily reduced by NaBH(4) or LiAlH(4). T...
Text Solution
|
- Carbon-oxygen double bond are easily reduced by NaBH(4) or LiAlH(4). T...
Text Solution
|