Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
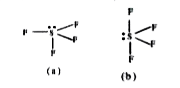
- For the molecule , Why is structure (b) more stable than structur...
Text Solution
|
- In which of the following first resonating structure is more stable th...
Text Solution
|
- A canonical structure will be more stable if
Text Solution
|
- The most stable canonical structure of this molecule is :
Text Solution
|
- In which of the following first resonating structure is more stable th...
Text Solution
|
- In which of the following pairs of resonating structures first resonat...
Text Solution
|
- The most stable canonical structure of this molecule is :
Text Solution
|
- यद्यपि फीनॉक्साइड की पाँच अनुनादी संरचनाएँ होती हैं, किन्तु यह दो अनुन...
Text Solution
|
- The most stable canonical structure of this molecule is
Text Solution
|