A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
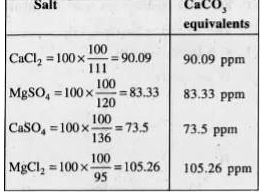
- Different samples of water containing each 100 ppm of CaCl(2),MgSO(4),...
Text Solution
|
- One litre of a sample of hard water contains 5.55 mg of CaCl(1) and 4....
Text Solution
|
- Determine the degree of hardness of a sample of water containing 30 pp...
Text Solution
|
- One litre of a sample of hard water contain 4.44mg CaCl(2) and 1.9mg "...
Text Solution
|
- One kilogram sample of hard water contains 4.44 mg of CaCl(2) and 1.9 ...
Text Solution
|
- 100g of a water samples is found to contain 12 mg of MgSO(4) calculate...
Text Solution
|
- The degree of hardness of a given sample of hard water is 40 ppm. If t...
Text Solution
|
- 1L of a sample of hard water contains 1 mg of each of CaCl(2) and MgCl...
Text Solution
|
- A sample of water containing KCL does not behave as hard water, but a ...
Text Solution
|