Text Solution
Verified by Experts
Topper's Solved these Questions
MECHANICAL PROPERTIES OF FLUIDS
KUMAR PRAKASHAN|Exercise SECTION-A Try Yourself (VSQs)|132 VideosMECHANICAL PROPERTIES OF FLUIDS
KUMAR PRAKASHAN|Exercise SECTION - B Numericals|29 VideosMACHANICAL PROPERTIES OF SOLIDS
KUMAR PRAKASHAN|Exercise QUESTION PAPER|11 VideosMOTION IN A STRAIGHT LINE
KUMAR PRAKASHAN|Exercise QUESTION PAPER|11 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-MECHANICAL PROPERTIES OF FLUIDS -QUESTION PAPER (SECTION -C)
- Give the definition of surface tension and formula in the context of (...
Text Solution
|
- Glycerine flows steadily through a horizontal tube of length 1.5m and ...
Text Solution
|
- A U- tube contains water and methlated spirit separated by mercury . ...
Text Solution
|
- What is the excess pressure inside a bubble of soap solution of radius...
Text Solution
|
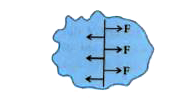 (i) Figure shown , on free surface of liquid if a line of unit length is out then the molecules one of side of line exert force on the molecules of other side of line perpendicular to the line and parallel to the surface then this force is known as surface tension (S). Here length
(i) Figure shown , on free surface of liquid if a line of unit length is out then the molecules one of side of line exert force on the molecules of other side of line perpendicular to the line and parallel to the surface then this force is known as surface tension (S). Here length