In 1905 Einstein proposed a new theory to explain photoelectric effect.
According to this theory,not only emission but absorption of energy (called phton) also takes place in discrete units.This unit are called quanta of enrgy of radiation.
Each quantum (photon)has energy hv,where v is frequency of radiation.Electron on surface will absorb hv energy.
If energy absorbed by electron on surface is more than minumum energy (work function `phi0`)then electron will be emitted with maximum kinetic energy.
Let energy of incident raidation is hv,maximum kinetic energy of electron is `k_(max)` and work function of metal is `phi0` then,
`phi0=hv_(0)`
`hv=K_(max)+phi0`
`thereforeK_(max)=hv-phi0`.....(1)
More tightly bound electrons will emerge with kineric enrgy less than maximum value.
With increase in intensity of light,number of electrons emitted per second will increase.
However maximum kinetic energy of electron emitted is determined by energy of photon.
In Einstein.s equation of photon.
In Einsteins.s equation maximum kinetic energy is `K_(max)=eV_(0)` where `V_(0)` is stopping potential from equation (1)
`therefore eV_(0)-hv-phi_(0)`........(2)
Here graph of enegry versus frequency is a straight line. `V_(0)=((h)/(e))v-(phio)/(e)`slope of `V_(0)tov`
graph is `(h)/(e)`. This does not depend on type of matter used.
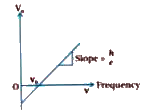
Figure shows graph of `V_(0)toV`
Einstein.s equation suggest that for phtoelectric emission when enrgy energy of incident radiation should be more than work function `hvgt phi0`
`therefore K_(max)=hv-phi0`
`K_(max)` should not be negative .
`therefore phi0=hv_(0)`
`therefore V_(0)=(phio)/(h)`..........(3)
Equation (3) suggest than more is work function frequency `(V_(0))` corresponding to minimum energy will be more.
No matter how intense is the radiation frequenct of incident ,radiation should be larger than threshold frequency.
