Resultant were obtained by superposition of waves of different wavelengths is called wave packet.
Matter wave associated with electron is not extended in inifinite space.It is a wave packet extended about a central wavelength.
In this condition `Deltax` is not infinite but it has some definite value which depend on range of wave packet.
Also a wave packet does not have definite wavelength but made up of no. of wavelengths about a central wavelength.
With description of wave packet with de-Broglie relation and Born.s probability interpretation reproduce the Heisenber.s uncertaintly principle exactly.
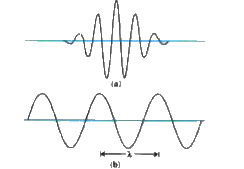
(a)The wave packet description of an electron.The wave packet corresponds to a spread of wavelength around some central wavelength (and hence by de Broglie relation ,a spread in momemtum).Consequently ,it is associated with an uncertainly in position `(Deltax)` and an uncertainly in momentum `(Deltap)`
(b) The matter wave corresponding to a definite momentum of an electrin extends all over space.In this case ,`Deltap=0` and `Deltaxtooo`
Figure (a) shows a schematic diagram of wave packet and figure (b) shows an extended wave will fixed wavelength.
The particle at that point.Probability density means probability per unit volume.Thus ,If A is the amplitude of the wave at a point ,`|A|^(2)DeltaV` is the probability of the particle being found in a small volume `DeltaV` around that point .Thus ,if the intensity of matter wave is large in a certain region,there is greater probability of the particle being found there than where the intensity is small.
 (a)The wave packet description of an electron.The wave packet corresponds to a spread of wavelength around some central wavelength (and hence by de Broglie relation ,a spread in momemtum).Consequently ,it is associated with an uncertainly in position `(Deltax)` and an uncertainly in momentum `(Deltap)`
(a)The wave packet description of an electron.The wave packet corresponds to a spread of wavelength around some central wavelength (and hence by de Broglie relation ,a spread in momemtum).Consequently ,it is associated with an uncertainly in position `(Deltax)` and an uncertainly in momentum `(Deltap)`