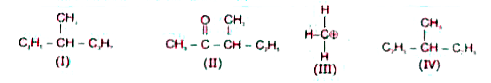A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ISOMERISM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE LEVEL II LECTURE SHEET (ADVANCED) More than one correct answer Type Questions|11 VideosISOMERISM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE LEVEL II LECTURE SHEET (ADVANCED) Linked Comprehension Type Questions (Passage - I)|3 VideosISOMERISM
AAKASH SERIES|Exercise PRACTICE SHEET EXERCISE-III (Stereo Isomerism) LEVEL-1I (ADVANCED) Integer Type Questions|4 VideosIONIC EQUILIBRIUM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL -II PRACTICE SHEET (ADVANCED) (Integer Type Questions))|8 VideosPERIODIC CLASSIFICATION
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (RECENT AIPMT/NEET QUESTIONS)|14 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ISOMERISM -ADDITIONAL PRACTICE EXERCISE LEVEL-I (MAIN) Straight Objective Type Questions
- Amongst the following compounds, the optically acitve alkane having lo...
Text Solution
|
- Which of the following compounds is not chiral ?
Text Solution
|
- Among the following four structures I to IV. it is true that
Text Solution
|
- Racemnic mixture is formed by mixing two
Text Solution
|
- Which of the following will have a meso-isomer also ?
Text Solution
|
- Which type of isomerism is shown by 2,3-dichlorobutane ?
Text Solution
|
- Which of the following molecules is expected to rotate the plane of po...
Text Solution
|
- The absolute configuration of
Text Solution
|
- Out of the following the alkene that exhibits optical isomerism is
Text Solution
|
- Which of the following statements best explain (s) the relationship be...
Text Solution
|
- Total number of stereoisomers of the following compound is CHCl=CH-CH(...
Text Solution
|
- Which of the following molecules can be optically active
Text Solution
|
- Butyne and Butadiene are
Text Solution
|
- The number of geometrical isomers of CH3CH=CH-CH-CH=CH-Cl
Text Solution
|
- Which of the following compounds can exhibit geometrical isomerism?
Text Solution
|
- A similarity between optical and geometrical isomerism is that
Text Solution
|
- Which of the following compounds exhibits stereo isomerism
Text Solution
|
- A minimum number of C atoms for a saturated hydrocarbon to exhibit opt...
Text Solution
|
- A compound with molecular formula, C7 H(16)shows optical isomerism, th...
Text Solution
|
- The number of enantiomers of the compound CH(3)CHBrCHBrCOOH is
Text Solution
|