A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -2(HYBRIDISATION AND VSEPR THEORY )|27 VideosCHEMICAL BONDING
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -2(MOLECULAR ORBITAL THEORY )|24 VideosCHEMICAL BONDING
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -2(IONIC BOND )|20 VideosATOMIC STRUCTURE
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (RECENT AIPMT/NEET QUESTIONS)|20 VideosCHEMICAL EQUILIBRIUM
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|30 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMICAL BONDING-OBJECTIVE EXERCISE -2(COVALENT AND DATIVE BOND )
- In which type of bond fomation, can a proton participate?
Text Solution
|
- The bonds present in [Cu(NH3)4] SO4 between copper and ammonia are
Text Solution
|
- How many water molecules present in CuSO(4).5H(2)O are hydrogen bonded...
Text Solution
|
- The element that exhibits neither electrovalency nor covalency is
Text Solution
|
- Octet rule is mostly violated in the compounds formed by
Text Solution
|
- In molecule, the formal charges of oxygen atoms 1,2,3 are respecti...
Text Solution
|
- Which of the following statemets in incorrect for PCl(5)?
Text Solution
|
- The attraction that non polar molecules have for each orther is primar...
Text Solution
|
- In bisulphate ion the formal charge on sulphur atom is
Text Solution
|
- The compound which contains both ionic and covalent bonds is
Text Solution
|
- Which of the following ion has maximum polarising power
Text Solution
|
- The bond between chlorine and bromine in BrCl is
Text Solution
|
- Oxygen cannot exhibit tetravalency and hexavalency like sulphur. This ...
Text Solution
|
- Which of the following is the most likely Lewis structure of nitroslyl...
Text Solution
|
- The following are some statements about the characteristics of covalen...
Text Solution
|
- Some statements about valence bond theory are given below (i) The st...
Text Solution
|
- The formal charges on the three oxygen atoms in O(3) molecule are
Text Solution
|
- In the electronic structure of acetic acid there are
Text Solution
|
- The formal charge of central oxyge atom in ozone molecule is
Text Solution
|
- Select the most stable structure of COCl(2)
Text Solution
|
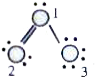 molecule, the formal charges of oxygen atoms 1,2,3 are respectively
molecule, the formal charges of oxygen atoms 1,2,3 are respectively