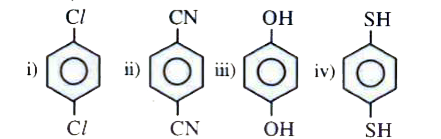
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -2(BOND CHARACTERS )|8 VideosCHEMICAL BONDING
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -3 (RECENT AIPMT/NEET QUESTIONS )|39 VideosCHEMICAL BONDING
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -2(HYDROGEN BOND )|17 VideosATOMIC STRUCTURE
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (RECENT AIPMT/NEET QUESTIONS)|20 VideosCHEMICAL EQUILIBRIUM
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|30 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMICAL BONDING-OBJECTIVE EXERCISE -2(DIPOLE MOMENT )
- Dipole moment of H2 X is 1.0 D. If the bond angle is 90^@, the approxi...
Text Solution
|
- The dipoleoment of HX is 1.2D. If the % ionic character of the bond is...
Text Solution
|
- Which bond angle theta would result in the maximum dipolemoment for th...
Text Solution
|
- From the following dipole moment (in Debye) values of methyl halides, ...
Text Solution
|
- A: Water molecule has zero dipole moment R: In water molecule dipole ...
Text Solution
|
- The dipole moment of BF(3) is zero because
Text Solution
|
- If a molecule MX(3) has zero dipole moment the sigma bonding orbitals ...
Text Solution
|
- Bent molecule having dipole moment among the following
Text Solution
|
- Which one of the following mu=0
Text Solution
|
- The difference of electronegativity between X and Y is 2. If X and Y f...
Text Solution
|
- Arrange in the order of increassing dipole moment I. toluene II. m...
Text Solution
|
- Whcin bond is most polar?
Text Solution
|
- The dipoleoment of HX is 1.2D. If the % ionic character of the bond is...
Text Solution
|
- The following are some statements about dipole moment. i. The dipole...
Text Solution
|
- Which of the following is more stable
Text Solution
|
- For which of the following molecule significat mu!=0?
Text Solution
|
- The table shown lists the bond dissociation energies (E("diss")) for s...
Text Solution
|
- The number and type of bonds between two carbon atoms in CaC(2) are
Text Solution
|
- From the following give statements of the order of dipolemoments. (i...
Text Solution
|
- KF combines with HF to form KHF(2). The compound contains the species
Text Solution
|