Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE- 1 (LONG ANSWER QUESTIONS)|2 VideosCHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE- 1 (SHORT ANSWER QUESTIONS)|13 VideosCHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (PRACTICS SHEET (ADVANCED) INTEGER TYPE QUESTIONS)|3 VideosCHEMICAL THERMODYANMICS
AAKASH SERIES|Exercise Questions For Descriptive Answers|28 VideosELECTRON MIGRATION EFFECTS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|10 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMICAL THERMODYNAMICS-PROBLEMS
- In a process, a system loses 125 J of heat when 400 J of work was done...
Text Solution
|
- A system absorbs 10kJ of heat at constant volume and its temperature r...
Text Solution
|
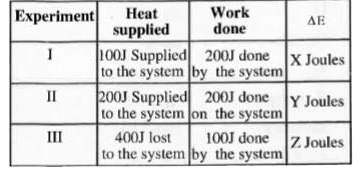
- From the observations given below, suggest the relation between X, Y a...
Text Solution
|
- What are the values of w and DeltaE, when a system absorbs 250J of he...
Text Solution
|
- C(("graphite")) + H2O((g)) to CO((g)) + H(2(g)), DeltaH = +131.4 kJ....
Text Solution
|
- N(2(g)) + O(2(g)) + 180.6 kJ to 2NO((g)), calculate (a) heat of reacti...
Text Solution
|
- DeltaH^(0) for a reaction F(2) + 2HCl rarr 2HF + Cl(2) is given as -35...
Text Solution
|
- H(2(g)) + 1/2O(2(g)) rarr H2O((l)) , DeltaH = -286.2KJ H2O((l)) rarr...
Text Solution
|
- Calculate the value of (DeltaH - DeltaE)in calories for the combustion...
Text Solution
|
- 19.5 g of benzene on burning in the free supply of oxygen liberated 12...
Text Solution
|
- 4.184g of benzoic acid was burnt in bomb calorimeter. The rise in the ...
Text Solution
|
- H(2)SO(4)(aq)+2NaOH((aq))to Na(2)SO(4)(aq) +2H(2)O((l)). Suggest the h...
Text Solution
|
- HCN((aq)) + NaOH((aq)) to NaCN((aq)) + H2O((l)) , DetlaH = -12.13 kJ. ...
Text Solution
|
- In the reaction between strong acid and strong base, 1.8 g of water is...
Text Solution
|
- mole of same solute is dissolved in 200 moles of water 18.58 kJ of hea...
Text Solution
|
- The bond enthalpies of D-Dand O-O and D-O are respectively, + 440, +49...
Text Solution
|
- DeltaH for the formation of XY is -200 kJ mol^(-1).The bond enthalpies...
Text Solution
|
- Heats of combustion of graphite carbon and carbon monoxide are respect...
Text Solution
|
- The heat of transition of S(alpha) into S(beta) is +2.5 kJ. The heat o...
Text Solution
|
- Heats of combustion of benzene, carbon and hydrogen are - 782, -94, -6...
Text Solution
|