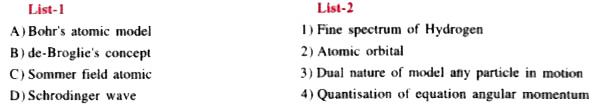A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
AAKASH SERIES|Exercise Level - I (EAMCET) (Exercise -IV (Quantum numbers))|21 VideosATOMIC STRUCTURE
AAKASH SERIES|Exercise Level - I (EAMCET) (Exercise - V (Electronic configuration , Aufbau principle, Pauli.s Exclusion principle and Hund.s rule ))|30 VideosATOMIC STRUCTURE
AAKASH SERIES|Exercise Level - I (EAMCET) (Exercise -II (Bohr theory))|20 VideosAROMATIC HYDROCARBONS
AAKASH SERIES|Exercise OBJECTIVE EXERCIES - 3 (RECENT AIPMT/NEET QUESTIONS)|10 VideosCHEMICAL BONDING
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -3 (RECENT AIPMT/NEET QUESTIONS )|39 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ATOMIC STRUCTURE-Level - I (EAMCET) (Exercise -III (De-Broglie.s Theory , Heisenberg Principle , Schrodinger equations & orbitals ))
- The momentum of a particle of wave length 1 Å is
Text Solution
|
- The de Broglie wavelength of a particle with mass 1 g and velocity 100...
Text Solution
|
- The de Broglie wave length of a riffle bullet of mass 2 grams moving w...
Text Solution
|
- A cricket ball of mass 0.5 kg is moving with a velocity of 100 ms^(-1)...
Text Solution
|
- If the Planck's constant h = 6.6 xx 10^(-34) Js, the de-Broglie wave l...
Text Solution
|
- The de Broglie wave length associated with a particle of mass 1 mg mov...
Text Solution
|
- According to Schrodinger model, nature of electron in an atom is as
Text Solution
|
- Which one of the following expressions represent the electron probabil...
Text Solution
|
- Radial part of the wave function depends on quantum numbers
Text Solution
|
- A given p-orbitals isfold degenerated
Text Solution
|
- Number of nodal planes that a p-orbital has
Text Solution
|
- Which of the following is correct with respect to .p. orbitals ?
Text Solution
|
- The maximum number of electrons accomodated in 5f orbitals
Text Solution
|
- The maximum probability of finding an electron of a particular energy ...
Text Solution
|
- Number of nodes spaces in 4s orbital is
Text Solution
|
- (A) : The p-orbital has dumb-bell shape (R) : Electron present in p-...
Text Solution
|
- The number of nodal planes .d. orbital has
Text Solution
|
- In the radial probability distribution curve for the 2s orbital of the...
Text Solution
|
- (A) There are two noda regions in 3s-orbital (R) : There is no nodal...
Text Solution
|
- Match the following columns
Text Solution
|