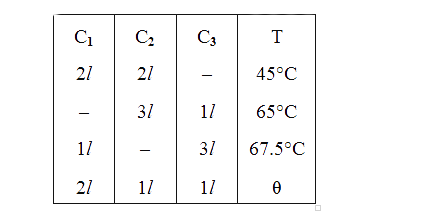Similar Questions
Explore conceptually related problems
Recommended Questions
- Three containers C1, C2 and C3 have water at different temperatures....
Text Solution
|
- Equal volumes of three liquids of densities rho1 , rho2 and rho3 , spe...
Text Solution
|
- Three liquids with masses m1 , m2 , m3 are throughly mixed. If their s...
Text Solution
|
- Two containers X and Y are filled with water at different temperature....
Text Solution
|
- Two beaker A and B contain water at different temperatures .When 1 lit...
Text Solution
|
- There are three containers C1, C2 and C3 filled with same material at ...
Text Solution
|
- Three containers C1, C2 and C3 have water at different temperatures....
Text Solution
|
- Three containers C(1), C(2) and C(3) have water at different temperatu...
Text Solution
|
- একটি পাত্রে 12°C তাপমাত্রার 40 g জল আছে। 80°C তাপমাত্রায় 50 g জল ওই ...
Text Solution
|