Similar Questions
Explore conceptually related problems
Recommended Questions
- The dipolemoment of are in order
Text Solution
|
- The dipolemoment of benzene is
Text Solution
|
- The dipolemoment of are in order
Text Solution
|
- Define Dipolemoment. Write its applications.
Text Solution
|
- what is dipolemoment
Text Solution
|
- The unit of dipolemoment is
Text Solution
|
- Dipolemoment is not zero for
Text Solution
|
- Which of the following has the highest dipolemoment ?
Text Solution
|
- The dipolemoment of benzene is
Text Solution
|
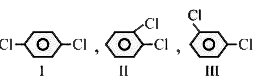 are in order
are in order