A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
AAKASH SERIES|Exercise Objective Exercise - 2|93 VideosCHEMICAL KINETICS
AAKASH SERIES|Exercise Objective Exercise - 3 (Previous NEET/AIPMT Questions)|32 VideosCHEMICAL KINETICS
AAKASH SERIES|Exercise Subjective Exercise-4 (Numerical Problems)|7 VideosCHEMICAL KINETCS
AAKASH SERIES|Exercise EXERCISE - 3.2|45 VideosCHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise Additional Practice Exercise|54 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMICAL KINETICS-Objective Exercise - 1
- For N(2)+3H(2)to2NH(3) rates of disappearance of N(2) and H(2) and rat...
Text Solution
|
- The reaction CH(3)COOC(2)H(5)+H(2)Ooverset(H^(+))(to) products is a/an...
Text Solution
|
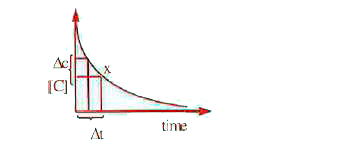
- From the graph the value (Deltac)/(Deltat) and the value of rate react...
Text Solution
|
- The rate of reaction which does not involve gases, does not depend upo...
Text Solution
|
- The specific rate constant of a reaction depends on the
Text Solution
|
- A catalyst accelerates the reaction, because
Text Solution
|
- The unit of rate constant depends on
Text Solution
|
- The temperature coefficient of a reaction is
Text Solution
|
- If concentration of reactants is made x times the rate constant k beco...
Text Solution
|
- The temperature coefficient of most of the reactions lies between
Text Solution
|
- For a reaction(K((1+10)))/(K((1)))=x. When temperature is increased fr...
Text Solution
|
- Increase of temperature will increase the reaction rate due to
Text Solution
|
- The rate constants of a reaction at 300K and 280 K respectively are K(...
Text Solution
|
- The rate constant K(1) of a reaction is found to be double that of ra...
Text Solution
|
- The Arrhenius equation expressing the effect of temperature on the rat...
Text Solution
|
- Activation energy depends on
Text Solution
|
- A catalyst in a chemical reaction does not change
Text Solution
|
- In general the rate of a given reaction can be increased by all the fa...
Text Solution
|
- The effect of temperature on a reaction rate for whilch Ea is zero is ...
Text Solution
|
- The rate expression gives the relation between rate of reaction and
Text Solution
|