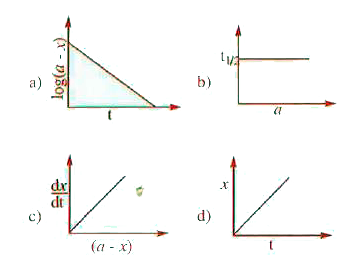
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
AAKASH SERIES|Exercise Objective Exercise - 3 (Previous NEET/AIPMT Questions)|32 VideosCHEMICAL KINETICS
AAKASH SERIES|Exercise Objective Exercise - 4 (Assertion (A) & Reason (R) Type Questions)|44 VideosCHEMICAL KINETICS
AAKASH SERIES|Exercise Objective Exercise - 1|124 VideosCHEMICAL KINETCS
AAKASH SERIES|Exercise EXERCISE - 3.2|45 VideosCHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise Additional Practice Exercise|54 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMICAL KINETICS-Objective Exercise - 2
- If (dx)/(dt)=k[H(3)O^(+)]^(n) and rate becomes 100 times when pH chang...
Text Solution
|
- The initial concentration of cane sugar is presence of an acid was red...
Text Solution
|
- 50% completion of a first order reaction takes place in 16 minutes. Th...
Text Solution
|
- For the process X(g)to products, (order ne 0), rates of disappearance...
Text Solution
|
- The temperature coefficient of a reaction is 2.5 . If its rate constan...
Text Solution
|
- A to B , K1= 0.693 sec^(-1) C to D, K2 = 0.693 "min"^(-1) If t1 and t2...
Text Solution
|
- For 2NH3(g) underset(Delta)overset(Pt)(to) products follows zero orde...
Text Solution
|
- The time needed for the completion of 2/3 of a 1st order reaction, whe...
Text Solution
|
- The rate constant of a first order reaction of 0.0693"min"^(-1). What ...
Text Solution
|
- Thermal decomposition of HI(g) on Gold surface follows Zero order kine...
Text Solution
|
- A to B and C to D are first order reactions. Ratio of t(99.9%) val...
Text Solution
|
- For a first order reaction temperature coefficient is 2. If the value ...
Text Solution
|
- Sucrose decompose in acid solution into glucose and fructose accordin...
Text Solution
|
- For an elementary reaction 2A+BtoC+D the active mass of B is kept con...
Text Solution
|
- The half-life period of a 1^(st) order reaction is 60 minutes. What pe...
Text Solution
|
- A particular reaction has a rate constant of 1.3xx10^(-2)s^(-1). Find ...
Text Solution
|
- For a general gaseous reaction of the type R to P , if the initial con...
Text Solution
|
- If the half life of a first order reaction is 60 min, the approximate ...
Text Solution
|
- Which of the following statement is incorrect ?
Text Solution
|
- Which of the following graphs represent a first order reaction (a=init...
Text Solution
|