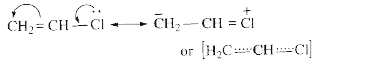Text Solution
Verified by Experts
Topper's Solved these Questions
ALKYL AND ARYL HALIDES
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE-1( SHORT ANSWER QUESTIONS)|5 VideosALKYL AND ARYL HALIDES
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE-1( VERY SHORT ANSWER QUESTIONS)|2 VideosALDEHYDES AND KETONES
AAKASH SERIES|Exercise Exercise 3.2|41 VideosAMINES AND AZO COMPOUNDS
AAKASH SERIES|Exercise PRACTICE SHEET - 6 (Integer answer type Questions)|9 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ALKYL AND ARYL HALIDES-OBJECTIVE EXERCISE - 4 (ASSERTION (A) & REASON (R) TYPE QUESTIONS)
- Why the chlorine atom in vinyl chloride is nonreactive?
Text Solution
|
- (A) Towards SN2 reaction, order of reactivity is CH3Br > CH3CH2Br > (C...
Text Solution
|
- (A) Pure chloroform does not give precipitate with AgNO3 solution. (...
Text Solution
|
- (A) Addition of bromine to 2-butene yields 2,3-dibromobutane. (R) Br...
Text Solution
|
- (A) Thionyl chloride reacts with primary alcohols to form pure alkyl h...
Text Solution
|
- (A) The boiling points of alkyl halides decrease in the order RI > RBr...
Text Solution
|
- (A) KCN reacts with methyl chloride to give methyl cyanide and methyl ...
Text Solution
|
- (A) In monohaloarenes, further electrophilic substitution occurs at or...
Text Solution
|
- (A) Benzonitrile is prepared by the action of chlorobenzene with KCN ...
Text Solution
|
- (A) Styrene on reaction with HBr gives 1-bromo-1-phenyl ethane. (R) ...
Text Solution
|
- (A) NBS is a specific reagent for allylic bromination (R) Allylic br...
Text Solution
|
- (A) Benzyl bromide when kept in acetone - water, produces benzyl alcoh...
Text Solution
|
- (A) 2-Bromobutane on reaction with sodium ethoxide in ethanol gives 1-...
Text Solution
|
- (A) Chloral reacts with phenyl chloride to form DDT (R) It is an ele...
Text Solution
|
- (A) CH3overset(Cl)overset(|)(C)HCH2CH3overset(Alc.KOH)(rarr)CH3CH=CHCH...
Text Solution
|
- (A) Addition of HBr on but-2-ene gives two structural isomeric product...
Text Solution
|
- (A) The nature of the solvent can influence the rotation of plane pola...
Text Solution
|
- (A) SN2 reactions are exothermic (R) SN2 reactions are thermochemica...
Text Solution
|
- (A) Chloroform can be used as general anaesthetic. (R) In presence o...
Text Solution
|
- (A) Ethyl chloride gives C2H5CN as major product with alc. KCN but C2H...
Text Solution
|