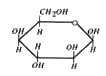
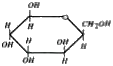
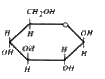
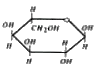A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CARBOHYDRATES
AAKASH SERIES|Exercise Level -II Lecture Sheet (Exercise-III)|4 VideosCARBOHYDRATES
AAKASH SERIES|Exercise Level -II Lecture Sheet (Exercise-IV)|4 VideosCARBOHYDRATES
AAKASH SERIES|Exercise Level -II Lecture Sheet (Exercise-I)|20 VideosBIOMOLECULES
AAKASH SERIES|Exercise OBJECTIVE EXERCISE-4 (ASSERTION (A) & REASON (R) TYPE QUESTIONS :)|84 VideosCARBOXYLIC ACIDS
AAKASH SERIES|Exercise PRACTICE SHEET-4 (Integer Type Questions)|9 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CARBOHYDRATES-Level -II Lecture Sheet (Exercise-II)
- A compound (X) C(6)H(12)O(6) is oxidised by bromine water into monobas...
Text Solution
|
- A compound (X) C(6)H(12)O(6) is oxidised by bromine water into monobas...
Text Solution
|
- Mutarotation is the change in specific rotation of an optically active...
Text Solution
|
- Mutarotation is the change in specific rotation of an optically active...
Text Solution
|
- Mutarotation is the change in specific rotation of an optically active...
Text Solution
|