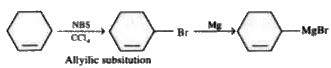
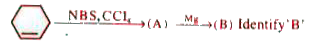A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Olefins can be halogenated in the allylic position by number of reagen...
Text Solution
|
- Assertion: NBS is a specific reagent for allylic bromination Reason: A...
Text Solution
|
- Allyl bromide and n-propyl bromide can bedistinguished by
Text Solution
|
- Which of the following reagents/tests cannot be used to distinguish al...
Text Solution
|
- Name a reagent used for the bromination at the allylic carbon atom.
Text Solution
|
- Reagent which cannot be used to distinguish allyl bromide from n-propy...
Text Solution
|
- Allylic bromination of an olefin is :
Text Solution
|
- Statement 1: NBS is a specific reagent for allylic bromination. Statem...
Text Solution
|
- Statement-1. NBS is a specific reagent for allylic bromination. Statem...
Text Solution
|