A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In which of the following C-X bond has highest bond dissociation entha...
Text Solution
|
- Which of the following has the highest bond dissociation enthalpy?
Text Solution
|
- Bond dissociation enthalpy and bond enthalpy are not the same for
Text Solution
|
- Which of the following is the correct order of bond dissociation entha...
Text Solution
|
- Which element-element bond has the highest bond dissociation energy ?
Text Solution
|
- Which of the following has highest bond dissociation energy ?
Text Solution
|
- Which mentioned bond has highest C-H bond dissociation energy.
Text Solution
|
- Which of the following C-H bond has the lowest bond dissociation energ...
Text Solution
|
- Which of the following has highest bond dissociation energy?
Text Solution
|
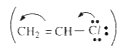 hence has highest bond dissociation energy
hence has highest bond dissociation energy