A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- product. The product mixture is treated with AgNO3 solution, correct s...
Text Solution
|
- 1- Phenoxypropane is treated with excess of conc. HI at 0^(@)C and the...
Text Solution
|
- The product formed when phthalimide is treated with a mixture of Br(2)...
Text Solution
|
- Product The correct statements about products is/are
Text Solution
|
- An mixture of two organic chlorine compounds was treated with sodium m...
Text Solution
|
- An mixture of two organic chlorine compounds was treated with sodium m...
Text Solution
|
- product. The product mixture is treated with AgNO3 solution, correct s...
Text Solution
|
- A mixture of two organic chlorine compounds was treated with sodium me...
Text Solution
|
- A mixture of two organic compounds was treated with sodium metal in et...
Text Solution
|
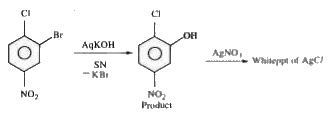
 product. The product mixture is treated with `AgNO_3` solution, correct statement is
product. The product mixture is treated with `AgNO_3` solution, correct statement is