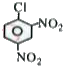
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Aryl halides are less reactive than alkyl halides due to the presence ...
Text Solution
|
- Aryl halides are less reactive towards nucleophilic substitution react...
Text Solution
|
- Why are aryl halides less reactive towards nucleophilic substitution r...
Text Solution
|
- Aryl halids are less reactive rtowards nucleophilic substitution react...
Text Solution
|
- Aryl halides are less reactive towards nucleophilic substitution react...
Text Solution
|
- Assertion: Aryl halides undergo nucleophilic substitution reactions wi...
Text Solution
|
- ऐरिल हैलाइड्स न्यूक्लियोफिलिक प्रतिस्थापन अभिक्रियाओं के प्रति ऐल्किल ...
Text Solution
|
- Aryl halide is less reactive than halide towards nucleophilic substitu...
Text Solution
|
- Aryl halides are less reactive than alkyl halides due to the presence ...
Text Solution
|