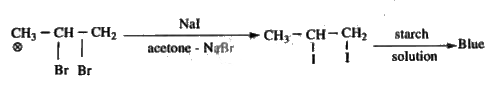A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A Compound 'X' has molecular formula C3H6Br2 reacts with Nal and ace...
Text Solution
|
- Ozone when reacts with potassium iodide solution liberates certain pro...
Text Solution
|
- Ozone when reacts with potassium iodide solution liberates certain pro...
Text Solution
|
- Which of the compounds with molecular formula C5H10 yields acetone on ...
Text Solution
|
- Which of the compounds with molecular formula C(5)H(10)yields acetone ...
Text Solution
|
- Solution x turned blue litmus to red and Solution y turned red litmus ...
Text Solution
|
- A substance X when heated with conc.\(H{2}SO{4}\) liberates a gas whic...
Text Solution
|
- आणविक सूत्र C(5)H(10) वाला निम्न में से कौन-सा यौगिक ओजोन से क्रिया कर...
Text Solution
|
- A compound X has molecular formula C3H4 One mole of X reacts with 2 mo...
Text Solution
|