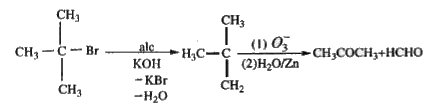
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- An unknown alkylhalide (A) reacts with alcoholic KOH to produce C4H8 ...
Text Solution
|
- An unsaturated hydrocarbon on complete hydrogenation gives 1-isopropl-...
Text Solution
|
- One mole of propanone and one mole of formaldehyde are the products of...
Text Solution
|
- An unknow alkyl 'A' reacts with alcoholic KOH to produce C(4)H(8). Ozo...
Text Solution
|
- An unknown alkyl halide A reacts with alcoholic KOH to produce a hydro...
Text Solution
|
- When one mole of an alkene on ozonolysis produces 2 moles of propanone...
Text Solution
|
- When one mole of an alkene or ozonolysis produces 2 moles of propanone...
Text Solution
|
- An unknown alkyl halide (A) reacts with alcoholic KOH to produce a hyd...
Text Solution
|
- एक मोल प्रोपेनॉन तथा एक मोल फार्मल्डिहाइड, एक मोल एल्कीन के ओजोनीअपघट...
Text Solution
|