A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The nucleophilic substitution reactions taking place in aromatic syste...
Text Solution
|
- In their nucleophilic substitution reactions, aryl halide resembles
Text Solution
|
- For a typical nucleophilic aromatic substitution reaction to take plac...
Text Solution
|
- For a typical nucleophilic aromatic substitution reaction to take plac...
Text Solution
|
- Which of the following cannot undergo nucleophilic substitution under ...
Text Solution
|
- Which the following compound undergoes nucleophilic substitution react...
Text Solution
|
- Which of the following compounds undergo nucleophilic substitution rea...
Text Solution
|
- Which of the following compounds undergoes nucleophilic substitution r...
Text Solution
|
- Alkanes undergo free radical substitution, aromatic compounds undergo ...
Text Solution
|
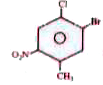 product. The solution is treated with `AgNO_3` solution. Which of the following is correct
product. The solution is treated with `AgNO_3` solution. Which of the following is correct 