A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
VIA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET - 3 (Linked Comprehension type questions)|6 VideosVIA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET - 3 (Match the following questions)|2 VideosVIA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET - 2 (Integer answer type Questions)|6 VideosVA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE EXERCISE|51 VideosVII GROUP ELEMENTS
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -4|55 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-VIA GROUP ELEMENTS-PRACTICE SHEET - 3 (Single or more than one option questions)
- 100 gms of 118% oleum reacted with excess water. How much H(2)SO(4) is...
Text Solution
|
- Mark the compound which gives carbon with conc. H(2)SO(4):
Text Solution
|
- The molecule of SO(2) is
Text Solution
|
- 2NaOH+SO(2) to A+H(2)O B+H(2)O+SO(2) to 2NaHSO(3). A and B are
Text Solution
|
- The behaviour of sulphur while reacting with water and alkali is simil...
Text Solution
|
- On passing SO(2) gas through an acidified solution of K(2)Cr(2)O(7)
Text Solution
|
- Which of the following can converts acidified Cr(2)O(7)^(2(-)) to gree...
Text Solution
|
- Which of the following statements are correct for SO(3) gas? I) It...
Text Solution
|
- 1) M.P. of Rhombic sulphur is higher than that of monoclinic sulphur ...
Text Solution
|
- Consider the following bond angles alpha= O-O-O inozone, beta=P-P-P in...
Text Solution
|
- Sulphur troxide can be obtained by which of the following reaction ?
Text Solution
|
- Which of the following shows wrong matching
Text Solution
|
- Which among the following are peroxo acid of sulphur?
Text Solution
|
- Oxidation number of sulpher in caro's acid
Text Solution
|
- Gas A+Na(2)CO(3)+H(2)O to B+C(gas) B+Na(2)CO(3) to D +C"(gas)"+H(2)O...
Text Solution
|
- Which of the following statements regarding thiosulphate ion is/are co...
Text Solution
|
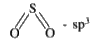 - `sp^(3)` hybridisation
- `sp^(3)` hybridisation