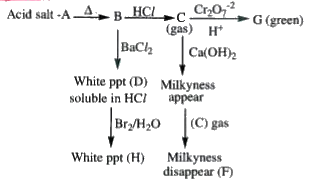
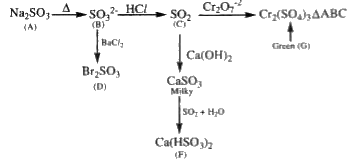
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
VIA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET - 5 (Match the following questions)|2 VideosVIA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET - 5 ( Integer answer type Questions)|6 VideosVIA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET - 4 (Integer answer type Questions)|2 VideosVA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE EXERCISE|51 VideosVII GROUP ELEMENTS
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -4|55 Videos
Similar Questions
Explore conceptually related problems