Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
VIA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET - 5 ( Integer answer type Questions)|6 VideosVIA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET - 5 (Linked Comprehension type questions)|3 VideosVA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE EXERCISE|51 VideosVII GROUP ELEMENTS
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -4|55 Videos
Similar Questions
Explore conceptually related problems
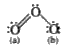 , Middle = 3, Oxygen (a) = 2, Oxygen (b) = 1
, Middle = 3, Oxygen (a) = 2, Oxygen (b) = 1 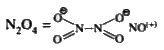 D) `H-overset(* *)N=overset(o+)N=overset(* *o+)(N: )`
D) `H-overset(* *)N=overset(o+)N=overset(* *o+)(N: )`